How to Determine How Much Excess Reactant Is Left
In one experiment 0866 mol of NO is mixed with 0503 mol of O2. B How much of the excess reactant is left.

Theoretical Actual Percent Yield Error Limiting Reagent And Excess Reactant That Remains Youtube Ch 8 Teaching Chemistry Chemistry Notes Chemistry
This gives a ratio in which no number is less than 1.

. Note that we might have reasonably assumed that iron metal was the limiting reactant since it was present in lesser amount in grams initially 100 g of Fe and 150 g of Cl 2. After heating the mass of the anhydrous compound is found to be 322 g. Nitric oxide NO reacts with oxygen gas to form nitrogen dioxide NO2 a dark brown gas.
2 is the limiting reactant. ADetermine the limiting reagent. 15 Limiting Reactant and Theoretical Yield - Cont.
The limiting reactant or reagent can be determined by two methods. Since lif is insoluble this is the final product. 431 322 109 g of water.
This gives you a molar ratio of Al to I_2 of 0044480009456 Usually you divide each number in the fraction by the smaller number of moles. 2NOg O2 2NO2. This is also an application of Le Chateliers principle.
This pattern of reaction is referred to as Markovinkov addition after the person 1 who first discovered that HBr adds in this way to a double bond. Calculate the molecular weight of each reactant and product. The intermediate carbocation is the tertiary carbocation rather than the primary carbocation that would be produced by addition to the CH 2 end of the double bond.
Na 2 CO 3--- 322 g 105988 gmol 00304 mol. The reactant that produces the lesser of the two amounts will tell you the limiting reactant. This solution will use dimensional analysis also called the unit-factor or unit-label.
This is a limiting reagent problem. If angle strain torsional strain or steric crowding in the reactant structure may is relieved by an alkyl or aryl shift to a carbocation site such a rearrangement is commonly observed. It is also wasteful.
More typically one reagent is completely used up and others are left in excess perhaps to react another day. B Calculate the number of moles of NO2 produced. In practice an excess of one reactant is used for two reasons.
The determination of the limiting reactant is typically just a piece of a larger puzzle. 1 Determine mass of water driven off. Iron metal is therefore in excess amount so there will be some Fe left over unreacted.
Determine the formula of the hydrate and then write out the name of the hydrate. Note that one product of the esterification reaction is water. Although a 3º-carbocation is initially formed the angle and torsional strain of the four-membered ring is.
You are expected to solve for the amount of product formed. Using the mole ration. In most limiting reactant stoichiometry problems the real goal is to determine how much product could be formed from a particular reactant mixture.
To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. 2 Determine moles of Na 2 CO 3 and water. The drawback in this method is that most of the excess reactant is left unreacted requiring extra steps to remove it.
The following examples illustrate rearrangements induced by the strain in a small ring. 1 To drive the reaction to completion 2 To maximize the yield of products The reactant that is added in excess amount is called the excess reactant while the one in lower or limiting amount is called the limiting reactant or limiting reagent. Convert the given amount of alloy reactant to solve for the moles of Fes reacted.
BaO 2 s 2HClaq --- H 2 O 2 aq BaCl 2 aq Solution. But it turned out that Cl 2 was the limiting reactant. Often it is straightforward to determine which reactant will be the limiting reactant but sometimes it takes a few extra steps.
We can classify many reagents as combinations of electrophile and. The question asks how much H2g was produced. A second method to drive the equilibrium towards product is to remove one of the products as it is formed.
Note that because so 2. Start with the compound you know the most about and use given ratios to convert it to the desired compound. Calculate the amount of product using each reactant.
How much excess reagent remains after the reaction is complete.
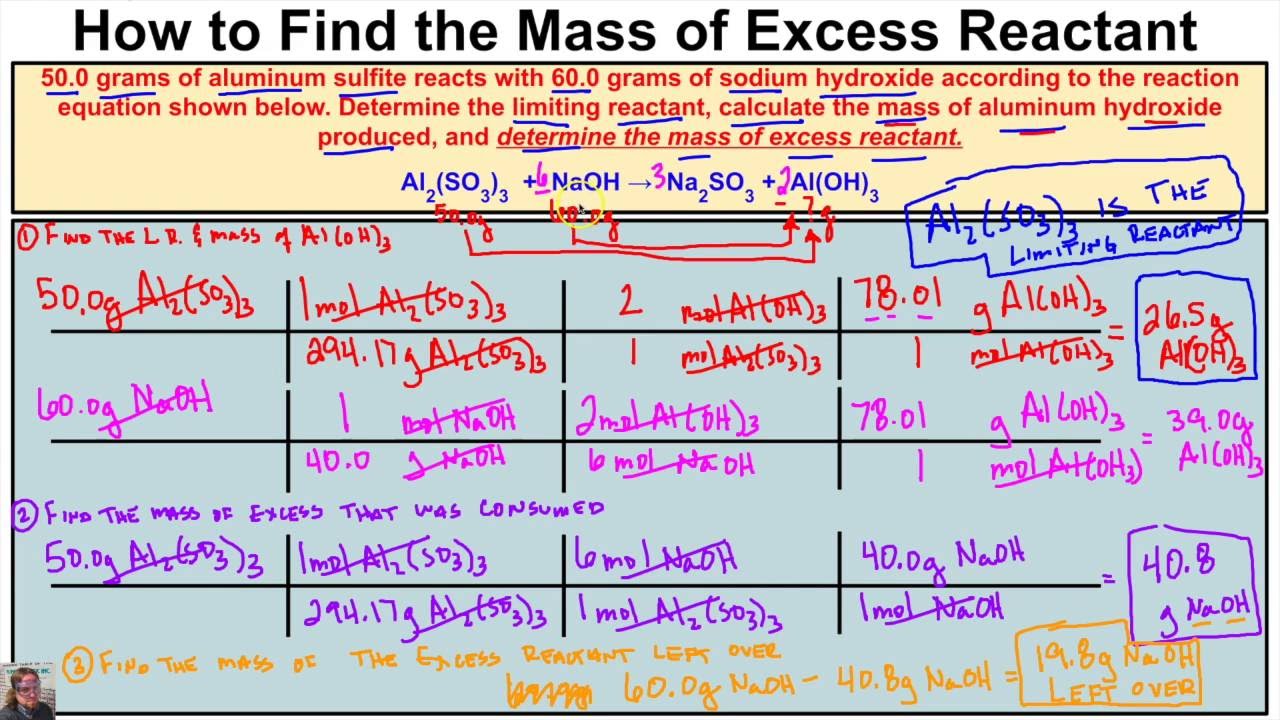
How To Calculate The Mass Of Excess Reactant Left Over In A Chemical Rea Chemistry Class Chemical Reactions Ap Chem
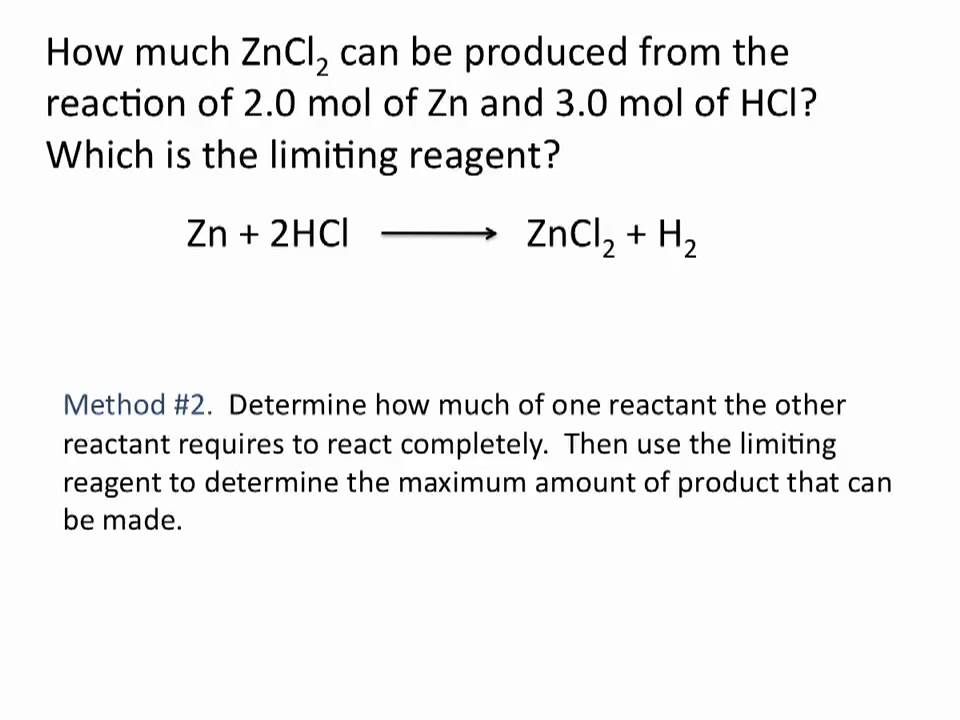
Limiting Reagent Chemistry Tutorial Youtube Chemistry Tutorial School

Introduction To Limiting Reactant And Excess Reactant Science Sciencewithtylerdewitt Tylerdewitt Tu Chemistry Help Apologia Chemistry High School Chemistry

Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry Study Help Chemistry Videos Tutorial
Comments
Post a Comment